Saif
Senior Member
- Joined
- Jan 24, 2024
- Messages
- 15,397
- Reaction score
- 7,874
- Nation

- Residence

- Axis Group


The promises and pressures of Bangladesh’s pharma industry
There is no doubt that Bangladesh’s pharmaceutical industry has shown remarkable potential.
The promises and pressures of Bangladesh’s pharma industry

VISUAL: ANWAR SOHEL
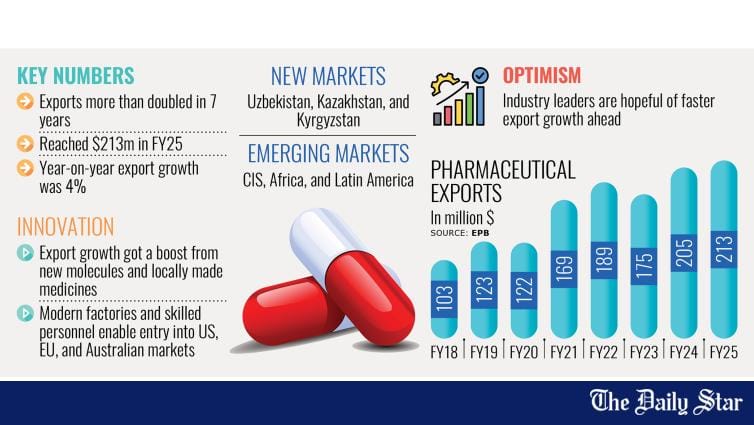
The pharmaceutical sector in Bangladesh, now valued at more than $3.5 billion (as of 2023), stands as one of the most compelling narratives of progress in the country's economic landscape. From meeting 98 percent of domestic medicine needs to reaching over 150 countries with exports, this industry has evolved into a cornerstone of both national health and economic resilience. Our pharmaceutical companies are not only providing affordable healthcare across the country, but they are also gradually earning recognition on the global stage. As we approach the country's graduation from the Least Developed Country (LDC) status in November 2026, there is a growing need for the industry to adapt to new global norms and challenges.
This transformation has not happened overnight. Decades ago, Bangladesh was heavily reliant on imported medicines. Today, it produces nearly all essential drugs domestically, including life-saving ones such as antibiotics, insulin, and cancer treatments. With a population exceeding 170 million, the demand for accessible and cost-effective healthcare continues to grow. The local pharmaceutical sector has stepped up in response, resulting in improved health outcomes and a strengthened national supply chain. Alongside this domestic impact, the country has extended its pharmaceutical reach to international markets. In FY2023-24, pharmaceutical exports were valued at $205.48 million, registering a 17.14 percent growth. Bangladeshi medicines reached regions in Africa, Southeast Asia, and even entered regulated markets like the United States and Europe. A key contributor to this success has been the industry's commitment to upgrading its technological capabilities and investing in more advanced manufacturing practices, including the production of biosimilars and complex generics.
Decades ago, Bangladesh was heavily reliant on imported medicines. Today, it produces nearly all essential drugs domestically, including life-saving ones such as antibiotics, insulin, and cancer treatments. With a population exceeding 170 million, the demand for accessible and cost-effective healthcare continues to grow. The local pharmaceutical sector has stepped up in response, resulting in improved health outcomes and a strengthened national supply chain.
Underlying this growth has been a strategic use of policy flexibility under the World Trade Organization's (WTO) Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement. Bangladesh currently benefits from a waiver that allows the production of generic versions of patented drugs without the need for costly licensing. This policy has provided a significant competitive edge in global markets. However, this advantage will come to an end after November 2026. Once the waiver expires, companies will be required to obtain a licence to produce patented medications. This shift is likely to increase production costs, potentially affecting the affordability of life-saving drugs. At the same time, global pharmaceutical giants may reclaim market space that Bangladeshi firms have started to occupy.
The expiration of the TRIPS waiver is just one aspect of a broader set of challenges that the industry will need to address. One of the most pressing issues is the overwhelming dependency on imported raw materials, specifically active pharmaceutical ingredients (API). More than 95 percent of these are sourced from countries like India and China, which makes the entire production chain vulnerable to disruptions and external price fluctuations. Beyond sourcing materials, the path to entering highly regulated export markets is filled with obstacles. Certifications for compliance with international standards can be expensive and time-consuming, often delayed further by bureaucratic inefficiencies at home. Building new manufacturing facilities or upgrading existing ones also tends to move slowly under the current administrative frameworks.
Another critical concern lies in the gap between the industry's technical demands and the available talent pool. Modern pharmaceutical manufacturing, especially in fields like biologics and hormone-based treatments, requires specialised training and a strong foundation in both research and engineering. Although progress has been made, there is still a long road ahead in terms of workforce development. Infrastructure development has not kept pace either. Projects like the long-awaited API Industrial Park have faced repeated delays, often tied to policy inconsistencies and administrative bottlenecks. As Bangladesh graduates from the LDC status, it is also set to lose various trade privileges, including duty-free access to several export markets. This shift may erode the cost competitiveness that has so far helped our pharmaceutical products find a foothold abroad.
Still, despite these hurdles, there is reason to be optimistic. While highly regulated markets have always attracted the most attention, there are numerous unregulated and semi-regulated markets in regions like Africa, Latin America, and parts of Asia that offer immediate opportunities. These areas typically have fewer entry barriers and a strong demand for affordable, quality medicines. Accelerating the establishment of the API Industrial Park and supporting local production capabilities can significantly reduce dependency on imported raw materials, making the industry more resilient and cost-effective in the long run. With global demographics shifting and an ageing population driving the need for drugs targeting chronic illnesses and cancer, Bangladesh has a timely opportunity to develop its expertise in biosimilars and niche therapeutic areas through targeted investment in research and development.
From being heavily dependent on imports to becoming a growing exporter of essential medicines, Bangladesh's pharmaceutical story is one of resilience and reinvention. As the country enters a more competitive and complex global trade environment, the industry must prepare to meet new expectations.
Collaboration between the public and private sectors will be key in unlocking the next phase of innovation. Government incentives, policy support, and funding for research facilities, when combined with private sector efficiency and ambition, can create a powerful engine for long-term growth. As part of this preparation, the government can also explore negotiating an extension to the TRIPS transition period to allow more time for adaptation. Building frameworks for patent access, including participation in global patent pools or voluntary licensing agreements, can help maintain the affordability and availability of newer medicines. Additionally, streamlining regulatory approval processes, simplifying land acquisition, and providing tax incentives for research activities would send a strong signal to investors and innovators alike.
There is no doubt that Bangladesh's pharmaceutical industry has shown remarkable potential. But in order to sustain this momentum, we will need forward-looking policies, strong coordination across stakeholders, and a willingness to confront the industry's structural weaknesses. This is a moment to align regulatory architecture, engineering talent, research capabilities, internal compliance, and business strategy for the greater good.
From being heavily dependent on imports to becoming a growing exporter of essential medicines, Bangladesh's pharmaceutical story is one of resilience and reinvention. As the country enters a more competitive and complex global trade environment, the industry must prepare to meet new expectations. With strategic investments, collaborative thinking, and confidence in our own capabilities, there is every reason to believe that Bangladesh can continue to grow as a global provider of affordable, high-quality medicine. On the other hand, the industry needs more proactive hand-holding and policy support from the government.
Mamun Rashid, an economic analyst, is chairman at Financial Excellence Ltd and founding managing partner of PwC Bangladesh. He has played a pivotal role in establishing pharmaceutical and healthcare facilities in Bangladesh's private sector over the last 35 years.
VISUAL: ANWAR SOHEL
The pharmaceutical sector in Bangladesh, now valued at more than $3.5 billion (as of 2023), stands as one of the most compelling narratives of progress in the country's economic landscape. From meeting 98 percent of domestic medicine needs to reaching over 150 countries with exports, this industry has evolved into a cornerstone of both national health and economic resilience. Our pharmaceutical companies are not only providing affordable healthcare across the country, but they are also gradually earning recognition on the global stage. As we approach the country's graduation from the Least Developed Country (LDC) status in November 2026, there is a growing need for the industry to adapt to new global norms and challenges.
This transformation has not happened overnight. Decades ago, Bangladesh was heavily reliant on imported medicines. Today, it produces nearly all essential drugs domestically, including life-saving ones such as antibiotics, insulin, and cancer treatments. With a population exceeding 170 million, the demand for accessible and cost-effective healthcare continues to grow. The local pharmaceutical sector has stepped up in response, resulting in improved health outcomes and a strengthened national supply chain. Alongside this domestic impact, the country has extended its pharmaceutical reach to international markets. In FY2023-24, pharmaceutical exports were valued at $205.48 million, registering a 17.14 percent growth. Bangladeshi medicines reached regions in Africa, Southeast Asia, and even entered regulated markets like the United States and Europe. A key contributor to this success has been the industry's commitment to upgrading its technological capabilities and investing in more advanced manufacturing practices, including the production of biosimilars and complex generics.
Decades ago, Bangladesh was heavily reliant on imported medicines. Today, it produces nearly all essential drugs domestically, including life-saving ones such as antibiotics, insulin, and cancer treatments. With a population exceeding 170 million, the demand for accessible and cost-effective healthcare continues to grow. The local pharmaceutical sector has stepped up in response, resulting in improved health outcomes and a strengthened national supply chain.
Underlying this growth has been a strategic use of policy flexibility under the World Trade Organization's (WTO) Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement. Bangladesh currently benefits from a waiver that allows the production of generic versions of patented drugs without the need for costly licensing. This policy has provided a significant competitive edge in global markets. However, this advantage will come to an end after November 2026. Once the waiver expires, companies will be required to obtain a licence to produce patented medications. This shift is likely to increase production costs, potentially affecting the affordability of life-saving drugs. At the same time, global pharmaceutical giants may reclaim market space that Bangladeshi firms have started to occupy.
The expiration of the TRIPS waiver is just one aspect of a broader set of challenges that the industry will need to address. One of the most pressing issues is the overwhelming dependency on imported raw materials, specifically active pharmaceutical ingredients (API). More than 95 percent of these are sourced from countries like India and China, which makes the entire production chain vulnerable to disruptions and external price fluctuations. Beyond sourcing materials, the path to entering highly regulated export markets is filled with obstacles. Certifications for compliance with international standards can be expensive and time-consuming, often delayed further by bureaucratic inefficiencies at home. Building new manufacturing facilities or upgrading existing ones also tends to move slowly under the current administrative frameworks.
Another critical concern lies in the gap between the industry's technical demands and the available talent pool. Modern pharmaceutical manufacturing, especially in fields like biologics and hormone-based treatments, requires specialised training and a strong foundation in both research and engineering. Although progress has been made, there is still a long road ahead in terms of workforce development. Infrastructure development has not kept pace either. Projects like the long-awaited API Industrial Park have faced repeated delays, often tied to policy inconsistencies and administrative bottlenecks. As Bangladesh graduates from the LDC status, it is also set to lose various trade privileges, including duty-free access to several export markets. This shift may erode the cost competitiveness that has so far helped our pharmaceutical products find a foothold abroad.
Still, despite these hurdles, there is reason to be optimistic. While highly regulated markets have always attracted the most attention, there are numerous unregulated and semi-regulated markets in regions like Africa, Latin America, and parts of Asia that offer immediate opportunities. These areas typically have fewer entry barriers and a strong demand for affordable, quality medicines. Accelerating the establishment of the API Industrial Park and supporting local production capabilities can significantly reduce dependency on imported raw materials, making the industry more resilient and cost-effective in the long run. With global demographics shifting and an ageing population driving the need for drugs targeting chronic illnesses and cancer, Bangladesh has a timely opportunity to develop its expertise in biosimilars and niche therapeutic areas through targeted investment in research and development.
From being heavily dependent on imports to becoming a growing exporter of essential medicines, Bangladesh's pharmaceutical story is one of resilience and reinvention. As the country enters a more competitive and complex global trade environment, the industry must prepare to meet new expectations.
Collaboration between the public and private sectors will be key in unlocking the next phase of innovation. Government incentives, policy support, and funding for research facilities, when combined with private sector efficiency and ambition, can create a powerful engine for long-term growth. As part of this preparation, the government can also explore negotiating an extension to the TRIPS transition period to allow more time for adaptation. Building frameworks for patent access, including participation in global patent pools or voluntary licensing agreements, can help maintain the affordability and availability of newer medicines. Additionally, streamlining regulatory approval processes, simplifying land acquisition, and providing tax incentives for research activities would send a strong signal to investors and innovators alike.
There is no doubt that Bangladesh's pharmaceutical industry has shown remarkable potential. But in order to sustain this momentum, we will need forward-looking policies, strong coordination across stakeholders, and a willingness to confront the industry's structural weaknesses. This is a moment to align regulatory architecture, engineering talent, research capabilities, internal compliance, and business strategy for the greater good.
From being heavily dependent on imports to becoming a growing exporter of essential medicines, Bangladesh's pharmaceutical story is one of resilience and reinvention. As the country enters a more competitive and complex global trade environment, the industry must prepare to meet new expectations. With strategic investments, collaborative thinking, and confidence in our own capabilities, there is every reason to believe that Bangladesh can continue to grow as a global provider of affordable, high-quality medicine. On the other hand, the industry needs more proactive hand-holding and policy support from the government.
Mamun Rashid, an economic analyst, is chairman at Financial Excellence Ltd and founding managing partner of PwC Bangladesh. He has played a pivotal role in establishing pharmaceutical and healthcare facilities in Bangladesh's private sector over the last 35 years.